PedvaxHIB: Haemophilus b Conjugate Vaccine (Meningococcal Protein Conjugate): patient information, prescribing information, ingredients, manufacturer, adverse reactions and side effects
Tuesday, April 11, 2017 by Gregory Van Dyke
http://www.naturalnewsreference.com/2017-04-11-pedvaxhib-haemophilus-b-conjugate-vaccine-meningococcal-protein-conjugate-patient-information-prescribing-information-ingredients-manufacturer-adverse-reactions-and-side-effects.html
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Liquid PedvaxHIB safely and effectively. See full prescribing information for Liquid PedvaxHIB.
See full insert sheet at this link at the Natural News Reference website.
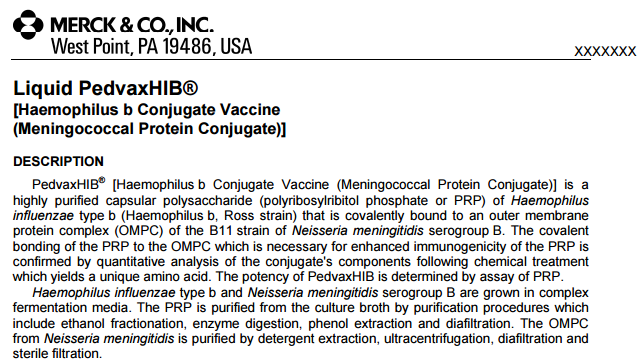
Liquid PedvaxHIB® [Haemophilus b Conjugate Vaccine (Meningococcal Protein Conjugate)]
Suspension for intramuscular injection
INGREDIENTS AND EXCIPIENTS
PedvaxHIB® [Haemophilus b Conjugate Vaccine (Meningococcal Protein Conjugate)] is a highly purified capsular polysaccharide (polyribosylribitol phosphate or PRP) of Haemophilus influenzae type b (Haemophilus b, Ross strain) that is covalently bound to an outer membrane protein complex (OMPC) of the B11 strain of Neisseria meningitidis serogroup B. The covalent bonding of the PRP to the OMPC which is necessary for enhanced immunogenicity of the PRP is confirmed by quantitative analysis of the conjugate’s components following chemical treatment which yields a unique amino acid. The potency of PedvaxHIB is determined by assay of PRP.
Haemophilus influenzae type b and Neisseria meningitidis serogroup B are grown in complex fermentation media. The PRP is purified from the culture broth by purification procedures which include ethanol fractionation, enzyme digestion, phenol extraction and diafiltration. The OMPC from Neisseria meningitidis is purified by detergent extraction, ultracentrifugation, diafiltration and sterile filtration.
Liquid PedvaxHIB is ready to use and does not require a diluent. Each 0.5 mL dose of Liquid PedvaxHIB is a sterile product formulated to contain: 7.5 mcg of Haemophilus b PRP, 125 mcg of Neisseria meningitidis OMPC and 225 mcg of aluminum as amorphous aluminum hydroxyphosphate sulfate (previously referred to as aluminum hydroxide), in 0.9% sodium chloride, but does not contain lactose or thimerosal. Liquid PedvaxHIB is a slightly opaque white suspension.
This vaccine is for intramuscular administration and not for intravenous injection. (See DOSAGE AND ADMINISTRATION.)
INDICATIONS AND USAGE
Liquid PedvaxHIB is indicated for routine vaccination against invasive disease caused by Haemophilus influenzae type b in infants and children 2 to 71 months of age.
Liquid PedvaxHIB will not protect against disease caused by Haemophilus influenzae other than type b or against other microorganisms that cause invasive disease such as meningitis or sepsis. As with any vaccine, vaccination with Liquid PedvaxHIB may not result in a protective antibody response in all individuals given the vaccine.
BECAUSE OF THE POTENTIAL FOR IMMUNE TOLERANCE, Liquid PedvaxHIB IS NOT RECOMMENDED FOR USE IN INFANTS YOUNGER THAN 6 WEEKS OF AGE. (See PRECAUTIONS.)
Revaccination
Infants completing the primary two-dose regimen before 12 months of age should receive a booster dose (see DOSAGE AND ADMINISTRATION).
DOSAGE AND ADMINISTRATION
Liquid PedvaxHIB FOR INTRAMUSCULAR ADMINISTRATION DO NOT INJECT INTRAVENOUSLY If there is an interruption or delay between doses in the primary series, there is no need to repeat the series, but dosing should be continued at the next clinic visit.
2 to 14 Months of Age
Infants 2 to 14 months of age should receive a 0.5 mL dose of vaccine ideally beginning at 2 months of age followed by a 0.5 mL dose 2 months later (or as soon as possible thereafter).
15 Months of Age and Older
Children 15 months of age and older previously unvaccinated against Hib disease should receive a single 0.5 mL dose of vaccine.
Booster Dose
In infants completing the primary two-dose regimen before 12 months of age, a booster dose (0.5 mL) should be administered at 12 to 15 months of age, but not earlier than 2 months after the second dose.
DOSAGE FORMS AND STRENGTHS
Inject 0.5 mL intramuscularly, preferably into the anterolateral thigh or the outer aspect of the upper arm. The buttocks should not be used for active vaccination of infants and children, because of the potential risk of injury to the sciatic nerve.
CONTRAINDICATIONS
Hypersensitivity to any component of the vaccine or the diluent.
Persons who develop symptoms suggestive of hypersensitivity after an injection should not receive further injections of the vaccine.
This monograph has been modified to include the generic and brand name in many instances.
WARNINGS AND PRECAUTIONS
As for any vaccine, adequate treatment provisions, including epinephrine, should be available for immediate use should an anaphylactoid reaction occur.
As for any vaccine, adequate treatment provisions, including epinephrine, should be available for immediate use should an anaphylactoid reaction occur.
Special care should be taken to ensure that the injection does not enter a blood vessel.
It is important to use a separate sterile syringe and needle for each patient to prevent transmission of hepatitis B or other infectious agents from one person to another.
ADVERSE REACTIONS
The use of Haemophilus b Polysaccharide Vaccines and another Haemophilus b Conjugate Vaccine has been associated with the following additional adverse effects: early onset Hib disease and Guillain-Barre syndrome. A cause and effect relationship between these side effects and the vaccination was not established.
USE IN SPECIFIC POPULATIONS
Pregnancy
Pregnancy Category C: Animal reproduction studies have not been conducted with PedvaxHIB. Liquid PedvaxHIB is not recommended for use in individuals 6 years of age and older.
Pediatric Use
Safety and effectiveness in infants below the age of 2 months and in children 6 years of age and older have not been established. In addition, Liquid PedvaxHIB should not be used in infants younger than 6 weeks of age because this will lead to a reduced anti-PRP response and may lead to immune tolerance (impaired ability to respond to subsequent exposure to the PRP antigen).49-51 Liquid PedvaxHIB is not recommended for use in individuals 6 years of age and older because they are generally not at risk of Hib disease.
Geriatric Use
This vaccine is NOT recommended for use in adult populations.
Revised:
https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM253652.pdf
http://naturalnewsreference.com/vaccine-insert-sheets/PedvaxHIB.pdf
Tagged Under: Tags: dosage, ingredients, insert sheet, pedvaxhib, side effects, usage, warnings